Inexpensive steroids reduce deaths of hospitalized Covid-19 patients, WHO analysis confirms
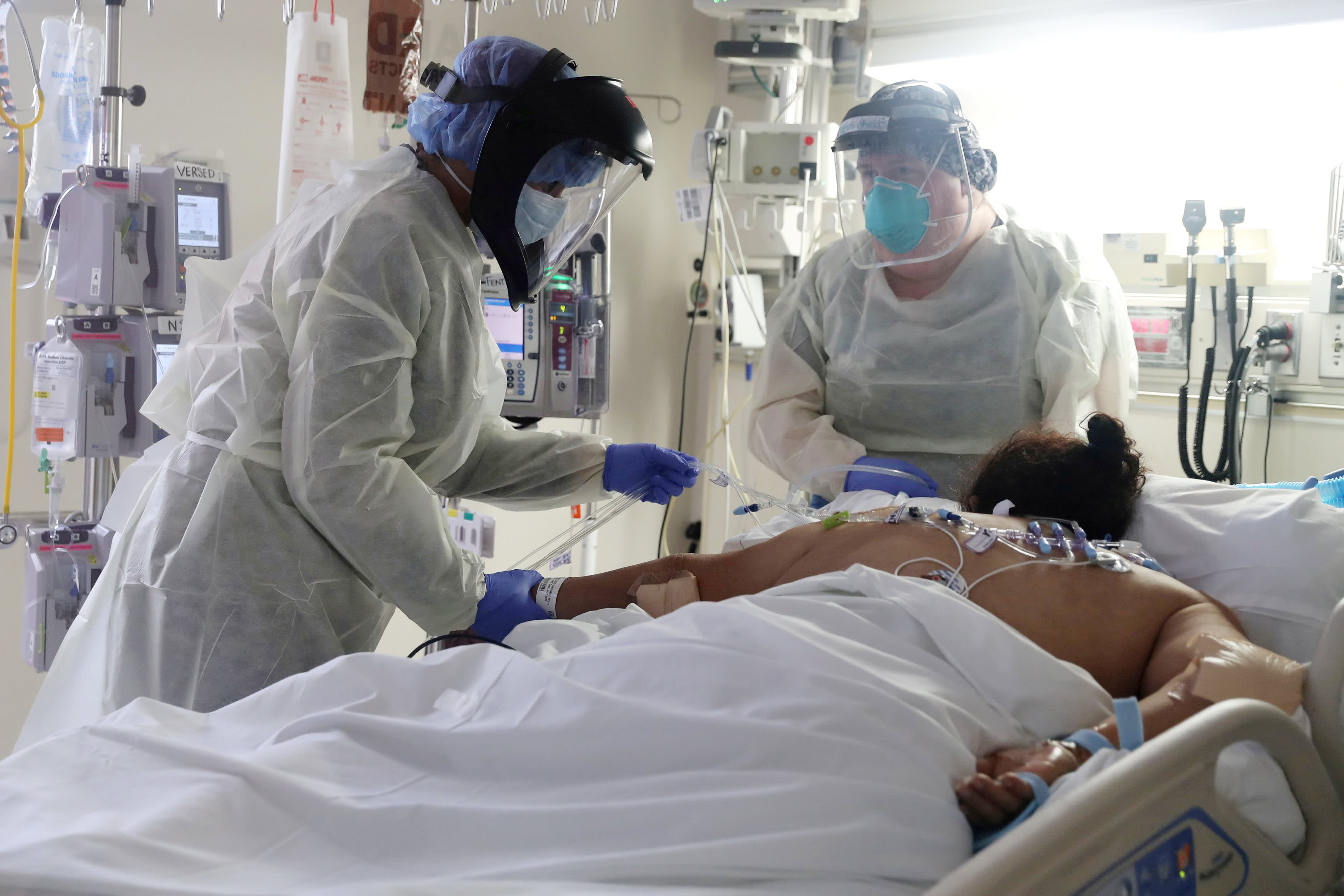
Use of inexpensive, readily available steroid drugs to treat people hospitalized with Covid-19 reduced the risk of death by one-third, according to an analysis encompassing seven different clinical trials conducted by the World Health Organization and published Wednesday in the Journal of the American Medical Association.
The positive steroid findings — the result of a pooled look at data known as a meta-analysis — confirm a similar survival benefit reported in June from a single, large study. Corticosteroids are the first, and so far only, therapy shown to improve the odds of survival for critically ill patients with Covid-19.
Based on the newly published data, the WHO on Wednesday issued new treatment guidelines calling for corticosteroids to become the standard of care for patients with "severe and critical" Covid-19. Such patients should receive 7-10 days of treatment, a WHO panel said. But it cautioned against use of the steroids in patients with non-severe illness, saying that "indiscriminate use of any therapy for COVID-19 would potentially rapidly deplete global resources and deprive patients who may benefit from it most as potentially life-saving therapy."
"The consistent findings of benefit in these studies provide definitive data that corticosteroids should be first-line treatment for critically ill patients with COVID-19," said Hallie Prescott and Todd Rice, professors of medicine at the University of Michigan and Vanderbilt University, respectively, in an accompanying JAMA editorial.
Nahid Bhadelia, medical director of the Special Pathogens Unit at the Boston University School of Medicine, said "there has been widespread adoption of steroids in the care of critically ill patients with Covid-19" since the first trial results in June. "This is particularly true in many resource-limited countries where I work. This meta-analysis adds further confidence" to those results, she added.
Other groups, including the National Institutes of Health and the Infectious Diseases Society of America, have already issued similar guidelines recommending the use of steroids to treat patients with severe Covid-19.
The new analysis included data on 678 patients randomized to treatment with steroids and 1,025 patients to usual care or a placebo. All of the patients had a confirmed diagnosis of Covid-19 and were admitted to the hospital. Most were on mechanical ventilation. Twenty-nine percent of the patients were women, but a breakdown by race was not disclosed.
After 28 days, 33% of the steroid-treated patients had died, compared to 41% of the patients on usual care or a placebo. In the meta-analysis, the difference in absolute mortality translated into a 34% reduction in the risk of death for those given steroids — a statistically significant result.
The survival benefit remained consistent regardless of the type of steroid administered, the dose, or whether patients were receiving mechanical ventilation or supplemental oxygen only, researchers found.
Eighteen percent of patients on steroids reported side effects compared to 23% of patients on usual care or placebo. Adverse events varied across trials but there was no suggestion that the risk of serious adverse events was higher in patients assigned to corticosteroids except for the two smallest trials, in which the total number of serious adverse events was one and three.
Corticosteroids do not directly attack the novel coronavirus. Instead, the drugs work by dampening the activity of a patient's immune system to prevent it from attacking the lungs — a serious and often fatal condition called acute respiratory distress syndrome, or ARDS.
The first evidence that common steroids could improve the survival of patients with severe Covid-19 came in June when British researchers conducting a large clinical trial called RECOVERY reported that the use of dexamethasone reduced the death rate by 35% in patients requiring ventilation and by 20% in patients who needed oxygen but were not ventilated.
Prior to the public announcement of the RECOVERY trial results, physicians had been reluctant to use steroids to treat severely ill Covid-19 patients due to concerns about side effects. Clinical trials involving other immune-suppressing drugs like IL-6 inhibitors were also yielding disappointing results.
Patrick Vallance, the U.K. government's chief scientific adviser, speaking in June, called the dexamethasone survival benefit from the RECOVERY study "tremendous news" and "a groundbreaking development in our fight" against Covid-19. But the findings also hampered efforts to confirm the results. Other randomized and controlled clinical trials investigating the use of steroids at that time were unable to enroll additional patients.
For that reason, the WHO's Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group stepped in to coordinate the meta-analysis of these incomplete but randomized and controlled trials. The analysis was done prospectively, meaning that data and outcomes from the seven individual trials were not known in advance, but were shared for the first time with the WHO team in order to reduce the chance of bias.
Three of the individual clinical trials of steroids were published in JAMA Wednesday, alongside the WHO's meta-analysis.
Source
Check Our More
No comments